DCP/MRP/RUP/SRP Slots
BASG as RMS
Please find below information about our available slots.
BASG will accept to act as RMS for as many submissions as possible within the limitation of its assessment resources. The routine of allocating time slots for marketing authorisation procedures (DCP, MRP, RUP, SRP) allows better scheduling of applications and optimisation of the use of the assessor resources.
Routine of allocating time slots
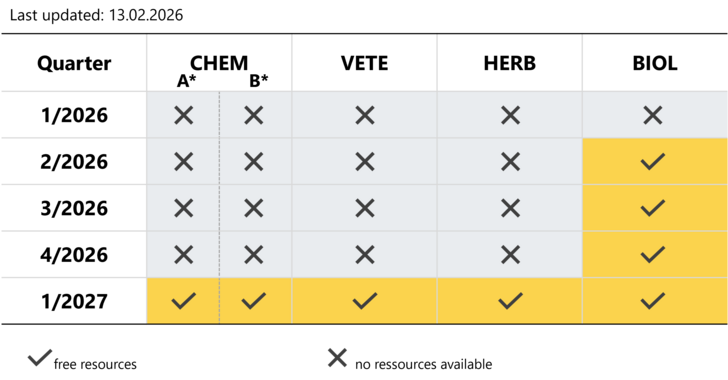
Please see below an availability-matrix showing available resources in the different assessor groups. The specific topics of the assessor groups are listed accordingly.
Slot-requests and -bookings can be placed at any time according to the availabilities as indicated in the below matrix.
Short-term available slots:
Due to late cancellations/postponements slots may become available at short notice. If you are interested in a short-term slot, please contact us for AT as RMS human via rms@basg.gv.at and for AT as RMS veterinary via vet.regulatory@basg.gv.at.
CHEM: Chemical medicinal products
*CHEM A: applications under article 10(1), 10(3), 10c (Dir. 2001/83/EC)
*CHEM B: applications under article 8(3), 10a, 10b (Dir. 2001/83/EC)
VETE: Veterinary medicinal products
HERB: Herbal medicinal products
BIOL: Biological medicinal products
Yellow boxes indicate resources are still available, grey boxes indicate that we will not be able to handle an application within the stipulated time tables.
For the application of time slots a completed request form needs to be submitted via the eServices or for AT as RMS human via rms@basg.gv.at and for AT as RMS veterinary via vet.regulatory@basg.gv.at.
The relevant request forms can be found in the Downloads section or on the CMD(h) website or the CMD(v) website.
Further information on the eServices can be found in the User Manual for Marketing Authorisation and Lifecycle Management of Medicines in the guidance section (New Application).
We will send an email of acceptance or refusal of a slot based on the decision of the particular groups of assessors to the contact person mentioned on the request form as soon as possible.
For RUPs and MRPs, please note that the compilation/update of the RMS Assessment Report starts with the submission of the update sequence in the agreed quarter.
General notes following the allocation of slots
To prepare the scheduled procedures, we will contact you via email in the quarter before the intended submission slot.
Applicants cannot change the active substance/therapeutic group or the time slot agreed by themselves. Applicants are advised to inform us for AT as RMS human via rms@basg.gv.at and for AT as RMS veterinary via vet.regulatory@basg.gv.at as early as possible if an agreed application cannot be submitted in due course to the slot agreed. Not meeting the agreed time slot without consultation with AGES MEA can lead to the loss of the slot and a new one needs to be requested.
Downloads
- Requested MS to act as RMS| 77 KBRequest for RMS in a DCP for medicinal products for human use29/09/2025
- Request for MRP/RUP for medicinal products for human use07/05/2025
- Appendix 1 to request for MRP/RUP for medicinal products for human use20/05/2020
- CMDv_Request_for_RMS| 41 KBRequest for MRP, SRP or DCP for medicinal products for veterinary use21/02/2022
For further information please contact
Human Medicinal Products: rms@basg.gv.at
Veterinary Medicinal Products: vet.regulatory@basg.gv.at
For general requests concerning DC-, Repeat Use-, SR- and MR-procedures please contact BASG via:
Tel. national: 050 555-36666
Tel. international: +43 (0)50 555-36666