FAQ Medicine shortages
1. Is there a possibility for distributors or an external service partner to assume the reporting obligation?
Local representatives of the marketing authorisation holders or distributors, which are either listed and clearly assigned in the package leaflet, or person(s) or companies authorised by the marketing authorisation holder, may apply for the role "notifier medicine shortage" in order to use the eServices "Authorisation and Lifecycle of Medicinal Products". For this purpose, please contact medicineshortage@basg.gv.at forwarding following documents:
- List of medicinal products to be supervised
- (either) Current package leaflet showing that applicants are listed as local representatives of the marketing authorisation holder.
- (or) Power of attorney issued by the marketing authorisation holder to the entrusted company to perform this function for dedicated medicinal products
2. Does the limited availability apply to each individual pack size? Does the notification have to be made if a different pack size is available?
A notification must be made as soon as a prescription-only medicine is limited available and the demand of patients is not met anymore. The notification and the data to be entered are always based on the marketing authorisation of the medicinal product and cover all pack sizes of the authorised medicinal product. In the course of the notification, the status of the availability ("not available", "limited available", "available", "not marketed") must be indicated for each pack size of the medicinal product. If not all pack sizes are marketed in Austria (e.g. there is no PIP code), the status for these shall be set to "not marketed".
3. How is "limited availability" to be defined?
A limited availability is considered to be a non-sufficient availability of a prescription-only medicine that does not meet demand and lasts at least four weeks. The adequate and continuous supply of the proprietary medicinal product for distribution by pharmacies or other licence holders for distribution in accordance with § 59 Austrian Medicines Act is not ensured during this period.
4. What is the meaning of the status "not available", "limited available", "available", "not marketed"
For each marketing authorisation of a medicinal product various pack sizes are approved. Not all of them are actually marketed in Austria.
"not available" means: The pack size of the medicinal product can no longer be dispensed by pharmacies in Austria.
"limited availability" means: The pack size of the medicinal product can no longer be supplied continuously and in sufficient quantities by pharmacies in Austria.
"available" means: The pack size of the proprietary medicinal product can be dispensed continuously and sufficiently by pharmacies in Austria.
"not marketed" means: The pack size of the medicinal speciality is approved, but has never been marketed in Austria.
5. How do you deal with medicinal products that are authorised but not marketed?
Not marketed pack sizes of a medicinal product must be notified as "not marketed".
However, if a medicinal product with all its approved pack sizes has never been marketed in Austria or the marketing cessation is permanent, a shortage-notification is not necessary, but you have to announce the marketing status in the eSercive "Marketing authorisation & lifecycle MP".
8. How long does the BASG have time to publish a not available or limited available medicinal product?
The processing within the BASG business hours shall always be carried out immediately. The processing time may be extended due to a high volume of notifications and the individual review of each notification. The notifications are always published in the public catalogues after successful processing by the BASG on the day after.
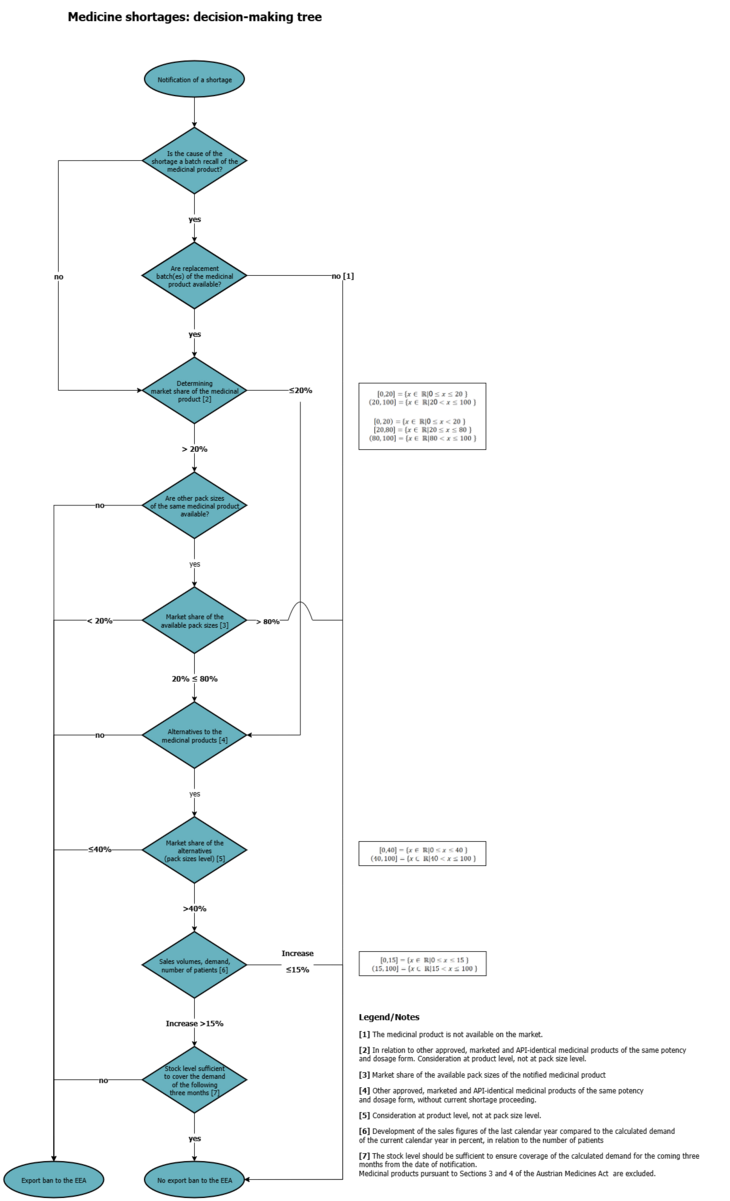
9. How does the BASG check whether an export ban to the EEA is applicable?
The review is carried out using the decision tree (see chart), which takes into account the mandatory fields "size of population affected by the shortage ", "market share", "market sales volume", "calculated patient need", "stock available at MAH" and "available potential alternative medicinal products".
10. Does the new reporting system also require old notifications already submitted before April 1, 2020 to be submitted again?
The old notifications are transferred to the new system. If it is necessary for the BASG to contact the marketing authorisation holder or the notifier, or vice versa, in order to examine a possible export ban to the EEA, the new mandatory fields "size of population affected by the shortage ", "market share", "market sales volume", "calculated patient need", "stock available at MAH" and "available potential alternative medicinal products" must be submitted subsequently.
12. What should be done if a marketing authorisation holder is able to supply sufficient quantities, but pharmacies and patients still report that the medicinal product is not available?
In this case § 4 para. 1 of the regulation on ensuring the provision of medicinal products applies. Please contact medicineshortage@basg.gv.at with a description of the facts of the case and do not make any prior notification via the eService portal or only in consultation with the BASG.
13. What is to be done if a marketing authorisation holder is no longer able to supply because the market leader has failed to supply?
In this case § 4 para. 1 of the regulation on ensuring the provision of medicinal products applies. Please contact medicineshortage@basg.gv.at with a description of the facts of the case and do not make any prior notification via the eService portal or only in consultation with the BASG.
14. What is to be done if a marketing authorisation holder has to set a quota for the goods in stock in order to ensure supply and avoid stockpiling or parallel exports?
In the case of a domestic quota of a prescription-only medicinal product for human use, a shortage notification must be reported and this circumstance has to be reported in the comment field of the reporting system in any case. In the case of a quota limited availability applies.
15. What is the procedure to be followed in the case of an export ban to the EEA if there are common packaging materials for the medicinal product for several countries (multi-country presentations)?
Packages with a "multi-country presentation" may not be exported from Austria to the EEA, as the authorisation holder intended them for Austria and placed them on the Austrian market.
16. What are the consequences of failing to report a medicine shortage?
If the BASG becomes aware that the marketing authorisation holder is not or not fully complying with its obligations under the regulation on ensuring the provision of medicinal products, the BASG must initiate an appropriate review by the authorities (see § 3 of the regulation on ensuring the provision of medicinal products). Pursuant to § 84 para. 1 no. 23 of the Austrian Medicines Act, a violation of the regulation on ensuring the provision of medicinal products constitutes an administrative offence and is punishable by a fine of up to 25,000 Euro, or up to 50,000 Euro in the event of recurrence.
17. Is the fee to be paid per notification, per medicinal product or per PIP code?
The fee has to be paid per notification of the proprietary medicinal product and not per pack size. The amount of the fee can be found in the current regulation of fees. The fee must be paid since July 1, 2020.
19. Are parallel imports and parallel distributions covered by the regulation on ensuring the provision of medicinal products?
Medicinal products having either a parallel import licence or a parallel distribution notice as a result of the European Medicines Agency (EMA) having conducted its check are not subject to the regulation on ensuring the provision of medicinal products.
20. What is meant by an export ban to the EEA?
According to § 5 para. 1 of the regulation on ensuring the provision of medicinal products, export to another contracting party of the European Economic Area is prohibited for reasons of public health protection. Excepted from this is the export for an individual named patient for the immediate prevention of a threat to life or serious damage to health ("Named Patient Use"). Third countries are exempt from the export ban to the EEA.
21. Is it necessary to report a restriction of supply if the patients' needs in Austria can be met by medicinal products from the EEA or a third country?
If there is a restriction of supply within the meaning of Section 1 (1) for the medicinal product authorised in Austria, a notification must be submitted to the BASG in any case, irrespective of any further shipment or import of medicinal products that were not originally intended for the Austrian market.
22. Must a prescription-only medicinal product that is stored in Austria for the issuance of a CPP (Certificate for a Pharmaceutical Product) be reported?
No notification of restriction in supply is necessary if a prescription-only medicinal product is stored in Austria for the issuance of a CPP (Certificate for a Pharmaceutical Product) and is not intended to meet the needs of patients in Austria.
23. What is to be done in case of a marketing cessation (temporary or permanent) for a medicinal product with an ongoing shortage notification?
With the amendment of the Austrian Medicines Act of February 14, 2022 and the associated change to Section 21 (2), the BASG must be notified of a temporary or permanent cessation of the marketing of proprietary medicinal products four months (instead of two months previously) in advance (see also the message in brief of Feb 14, 2022). The notification must (as previously) be submitted via the eServices and published by the BASG in accordance with Section 21 (4) Austrian Medicines Act.
For already submitted shortage notifications this means:
- In the event of a permanent marketing cessation, the shortage can be removed from the registers. The administrative procedure will be discontinued.
- In the case of a temporary marketing cessation, the existing marketing restriction remains in place or must be newly created. Please submit a statement of facts to medicineshortage@basg.gv.at.
24. Is a shortage to be notified if there is a temporary or permanent marketing cessation?
- No shortage notification is necessary for a permanent marketing cessation.
- A shortage notification is necessary for a temporary marketing cessation.
| Shortage notification | Notification of marketing cessation | |
| Permanent marketing cessation | no | yes |
| Temporary marketing cessation | yes | yes |
| No marketing cessation, but shortage | yes | no |
25. What should be done if there is a change of the marketing authorisation holder or the local representative during an ongoing shortage notification?
There is no automatical transfer of a changed marketing authorisation holder or the role “notifier medicine shortage” into the ongoing shortage notification procedure. Please contact medicineshortage@basg.gv.at about the change of the marketing authorisation holder, local representative and role “notifier medicine shortage”, respectively. Furthermore, a proper transfer of the current procedure to the new marketing authorisation holder including all necessary information for the correct handling of the shortage notification must be ensured.
