FAQ online services
1. What are the advantages of using the eService " Authorisation and Lifecycle MP "? Is it mandatory to use this eService?
The eService "Authorisation and Lifecycle MP" facilitates communication between Applicants/MAHs and the Austrian Federal Office for Safety in Health Care (Bundesamt für Sicherheit im Gesundheitswesen, BASG)/AGES Austrian Medicines and Medical Devices Agency (AGES Medizinmarktaufsicht) in procedures for marketing authorisation of medicinal products and their lifecycle management. All procedures are listed centrally whereas deficiency letters are attributed to a certain procedure. Thus it is easy to submit responses.
Even though use of the eService "Authorisation and Lifecycle MP" is not mandatory for now, communication as of 1 July 2013 will be primarily via this platform.
3. Are administrative decisions (Bescheide) electronically delivered via the eService?
Are administrative decisions (Bescheide) electronically delivered via the eService?Administrative decisions are electronically delivered via accredited delivery services in line with the Delivery Act (Zustellgesetz). For further information please refer to the website of the Federal Chancellery of the Business Service Portal.
4. Which situations require documents to be provided via the eService "Authorisation and Lifecycle MP"?
The eService "Authorisation and Lifecycle MP" is intended for the submission of product information texts for Austria and any related communication.
Submission of product information texts for Austria
The product information texts for Austria should be submitted either as working documents (WD) or - if Austria is the only member state concerned (national phase in MR-/DC-procedures, and national procedures in general) - via the eService "Authorisation and Lifecycle MP".
The eService can be used for proposed and final texts.
Please submit the dossiers/parts of dossiers as follows:
- Submission of dossiers
For the submission of dossiers in national or MR-/DC-procedures please continue to use the Common European Submission Platform (CESP) or DVD/CDs.
- Submission of responses to dossiers
For the submission of responses to dossiers please continue to use the Common European Submission Platform (CESP) or DVD/CDs.
5. How should I submit my product information texts to the Austrian Medicines and Medical Devices Agency (AGES Medizinmarktaufsicht) during the procedure?
Please use the eService "Authorisation and Lifecycle MP " for procedures that involve AT only (national phase in MR-/DC-procedures, and national procedures in general). Please do not use ZIP files!
If other member states are concerned as well (during MR-/DC-procedures), we recommend using the Common European Submission Platform (CESP), as the texts can be submitted to several member states at once.
6. Do I have to check the eService actively for new documents or do I receive an email as soon as a new document is uploaded?
A notification email is sent when a procedure is started, when a new communications entry is creaed and when a procedure is closed. The receiver list can be edited by the organisation's administrator. For further information see:
11. How can I provide a consultant with a restricted access to the eService "Authorisation and Lifecycle MP"?
Two different scenarios can be distinguished:
- Scenario A - Staff members of a consultant company should be provided with access to ALL pending procedures of an organisation.
Staff members of a consultant company should be included as staff members of the organisation in the “Administration of users and user rights” (Benutzer- und Rechteverwaltung).
- Scenario B - Staff members of a consultant company should be provided with access to CERTAIN pending procedures ONLY.
The consultant company has to be stated in the Application Form as “Applicant” or “Person/company authorised for communication on behalf of the Applicant during the procedure/after authorisation”. Staff members of consultant companies should be assigned within the consultant company ONLY.
12. Does my company need a local contact in Austria to register for the eService "Authorisation and Lifecycle MP"?
As you can register your company via the Internet using a web browser, a local contact person in Austria is not necessary. Further information can be found in the FAQ Registration and portal. You will also find instructions there.
After successful registration, the administrator of the organization will receive a document with the access data. The BASG/AGES Medical Market Supervisory Authority allows you to appoint one administrator per organization. The administrator can then give other members of his organization access to all pending procedures of the organization.
13. Does my company need a local contact in Austria in order to register for the eService "Authorisation and Lifecycle MP"?
As you can register your company via Internet using the web browser, a local contact in Austria is not necessary. For further information please refer to FAQ. Guidance notes (incl. translations of the German input fields in the registration form) are available.
After having registered successfully, the administrator of the organisation will receive a document containing the access data. The BASG/AGES Medizinmarktaufsicht allows you to appoint one administrator per organisation. The administrator can subsequently provide additional members of his organisation with access to all pending procedures of the organisation.
14. Does the BASG/AGES Medizinmarktaufsicht contact me via the eService"Authorisation and Lifecycle MP" to discuss my product information texts for Austria?
The eService "Authorisation and Lifecycle MP" is used for any communication during the national phase of MR-/DC-procedures as well as in national procedures.
16. My procedure is not listed in the eService
Please make sure that you have chosen the right organisation after registration. If you are not able to select this company, please contact the administrator of the respective company.
If you sent the procedure via CESP less than 7 days ago, it is possible that the procedure has not yet been created. Please have a little patience, your procedure will be created in the system as soon as possible.
If the submission via CESP has been longer than 7 days, please contact the processor according to the allocation scheme to clarify why the procedure is not displayed. Please enter the following information:
- Login (e-mail address)
- Organization No. and Name
- Name of the medicinal speciality
- CESP Submission ID
- approval number
- submission date
- EU procedure number
17. New column "Risk Class" shows no value
Since 13.10.2021, a new column called "Risk Class" can be found in the "Overview Medicinal Products" in the eService "Authorisation and Lifecycle MP". This column is currently still empty and serves to prepare for a later display of the risk classification with regard to the supply criticality of the respective medicinal product. Further information will be provided in due time.
18. Why is the ZIP folder empty for my completed procedure?
The ZIP folder that is created when you click on the "download zip" button contains the Summary of Product charactristics (SmPC) and package leaflet, barrier-free Summary of Product charactristics (SmPC) and package leaflet, labelling and mock-ups approved with the selected procedure, as well as the approval letter, the decision letter, the PAR (Public Assessment Report), the final statement on the national PSUR and the final statement on the national PSUR cycle change, if applicable. If none of these documents were approved or generated during the procedure, the ZIP folder will be empty.
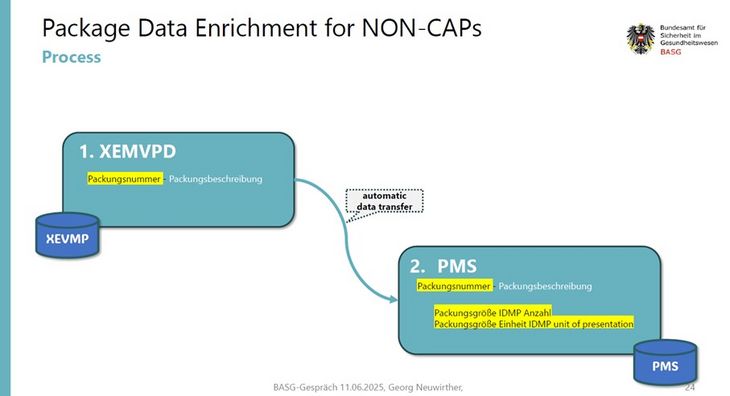
20. What is the export of the packaging data provided for?
The BASG/AGES MEA provides packaging data to support the necessary enrichment of packaging data in XEVMPD and PMS.
We recommend to add the “Packungsnummer” to the beginning of the package description.
recommended syntax: Packungsnummer -Beschreibung
Example: 957917002 -Tabletten in Streifen (Papier-PE-Aluminium-Copolymerfolie)
This makes it easier for MAHs and the BASG/AGES MEA to identify the packages transferred to PMS.
The use of “Packungsnummer” is not mandatory.