Non CE-labelled in vitro diagnostic medical devices New
The Federal Office for Safety in Health Care (BASG) warns against the use of the following in-vitro diagnostics:
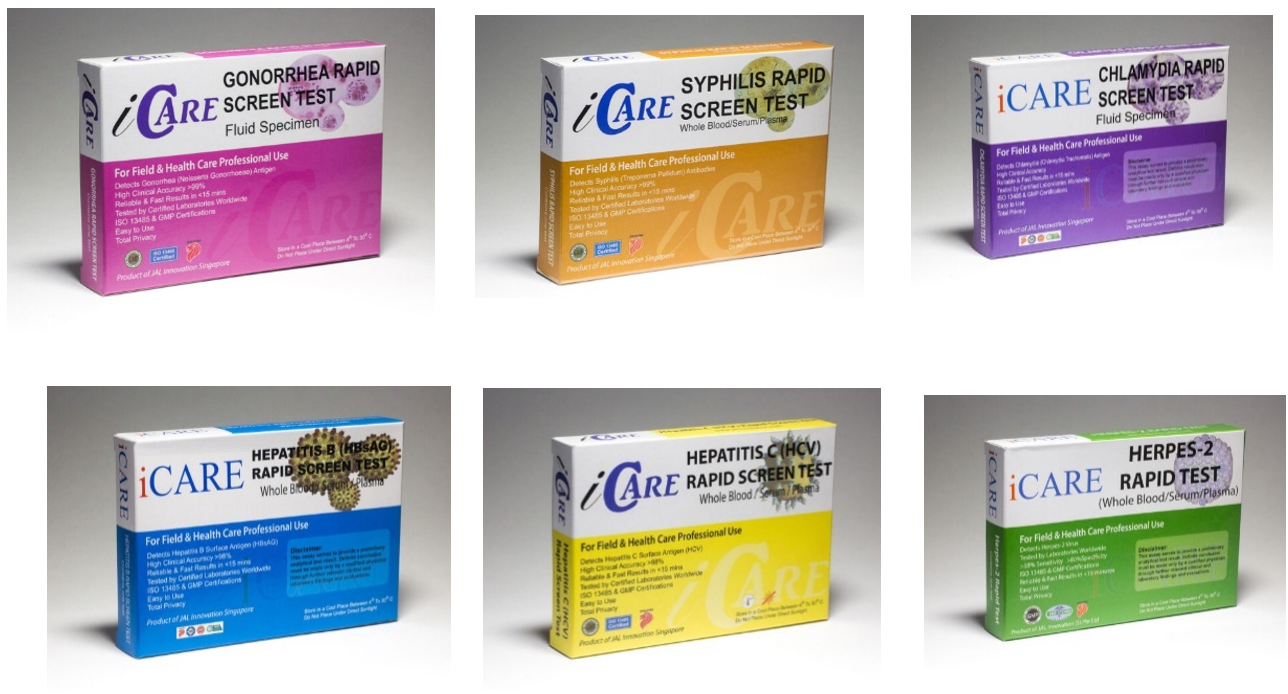
- iCare Syphilis Test
- iCare Hepatitis B Test
- iCare Hepatitis C Test
- iCare Chlamydia
- iCare Gonorrhoea
- iCare Herpes
These self-administered tests for the detection of sexually transmitted diseases (STDs) manufactured by Genesign Biotech (XIAMEN) Co., Ltd are also marketed under the brand name JAL Medical (Singapore) Pte. Ltd.
These products do not bear a CE mark, which means that they do not fulfil the requirements for in-vitro diagnostics. The BASG has information that the manufacturer has not carried out the required EU conformity assessment procedure. These products are therefore not marketable on the EU market.
The safety of these in vitro diagnostic medical devices has not been proven, which means that there is an unknown risk of false results.
At present, it cannot be ruled out that other "iCare" tests are also affected.
Affected users of these products are advised not to rely on the results, but to retest with a CE-marked in vitro diagnostic.
If necessary, consult your doctor.
Further information on how to recognise compliant in vitro diagnostic medical devices can be found at Medical devices - BASG
| Manufacturer | Genesign Biotech (Xiamen) Co., Ltd. Unit 03, 8th Floor, Building B14, Xiamen Biomedical Industrial Park 2076 Wengjjao West Read, Haicang District, Xiamen 361026, P.R. or JAL Medical (Singapore) Pte. Ltd. |
|---|---|
| Batch number(s) | all |
| Further information Link | https://instihivtest.com/en/safety-warning-icare-jal-medical-self-tests-not-ce-marked/ |