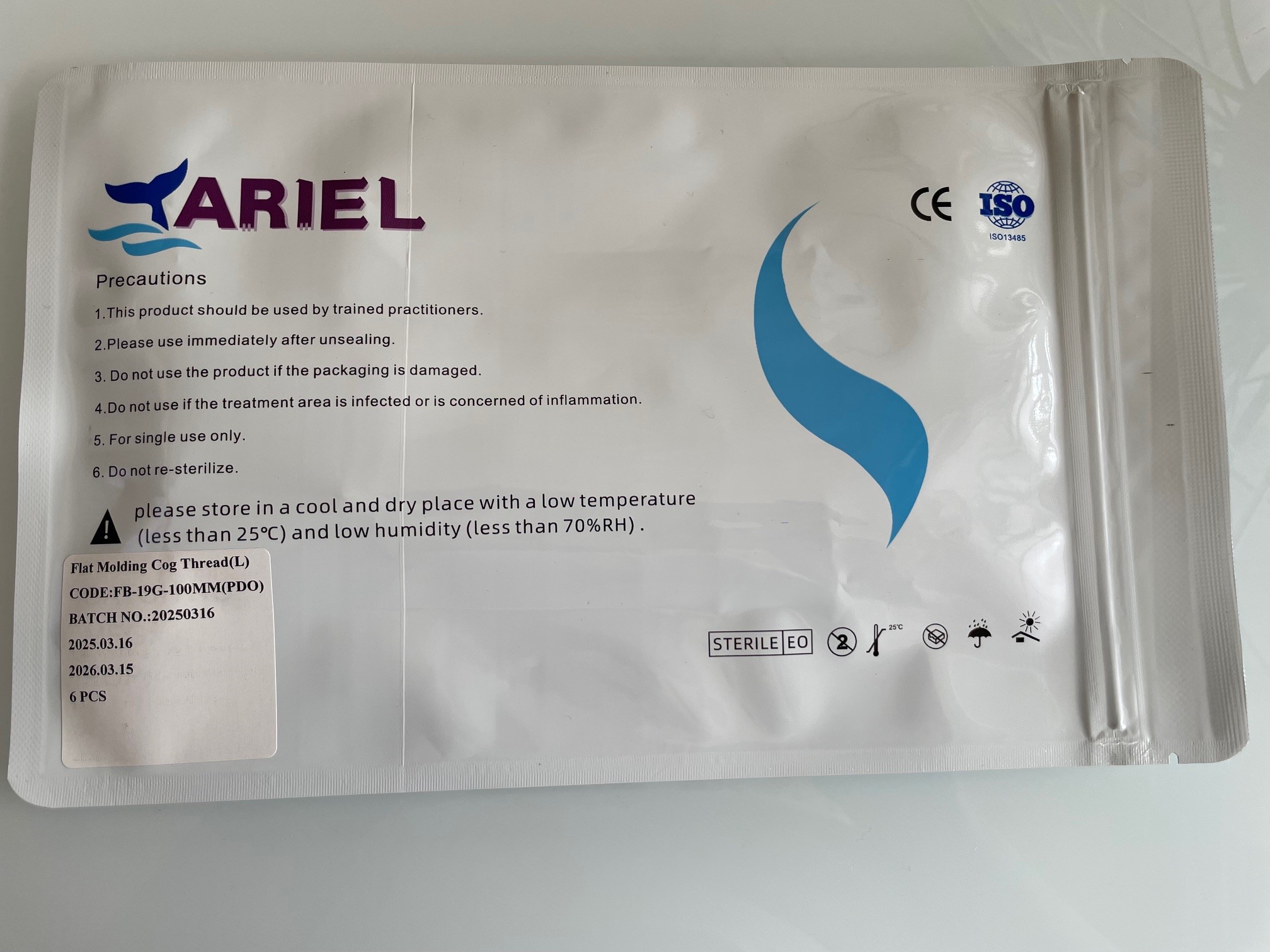
Safety Information Regarding “ARIEL PDO Threads” and Cannulas for Facelifting
The Federal Office for Safety in Health Care (BASG) warns of non-conforming subcutaneous and absorbable PDO threads, marketed as “ARIEL PDO Threads”, along with associated cannulas intended for mechanical tightening of loose and sagging skin (facelifting).
It has been determined that the “ARIEL PDO Threads” and associated cannulas do not comply with the regulatory requirements, and safe use of the respective products cannot be ensured. There is no information about the manufacturer or authorized representative on the affected products.
To date, BASG has received no reports of cases of damage or any other adverse events in Austria.
The products are advertised and can also be purchased through social media platforms.
BASG recommends purchasing medical devices exclusively from trusted sources and with knowledge of the respective manufacturers.
| Medical devices | ARIEL Molding Cog Thread (FB-19G-100MM(PDO)) with Subdeep Cannula Duck Bill |
|---|---|
| Manufacturer | Unknown |
| Distributor | ARIEL INTERNATIONAL TRADING., LTD Various accounts via social media |
| Serial number | UDI-DI - Unknown |