Safety information regarding the counterfeit medical device "Nasal Cannula, Luer-Lock F, PVC, adult"
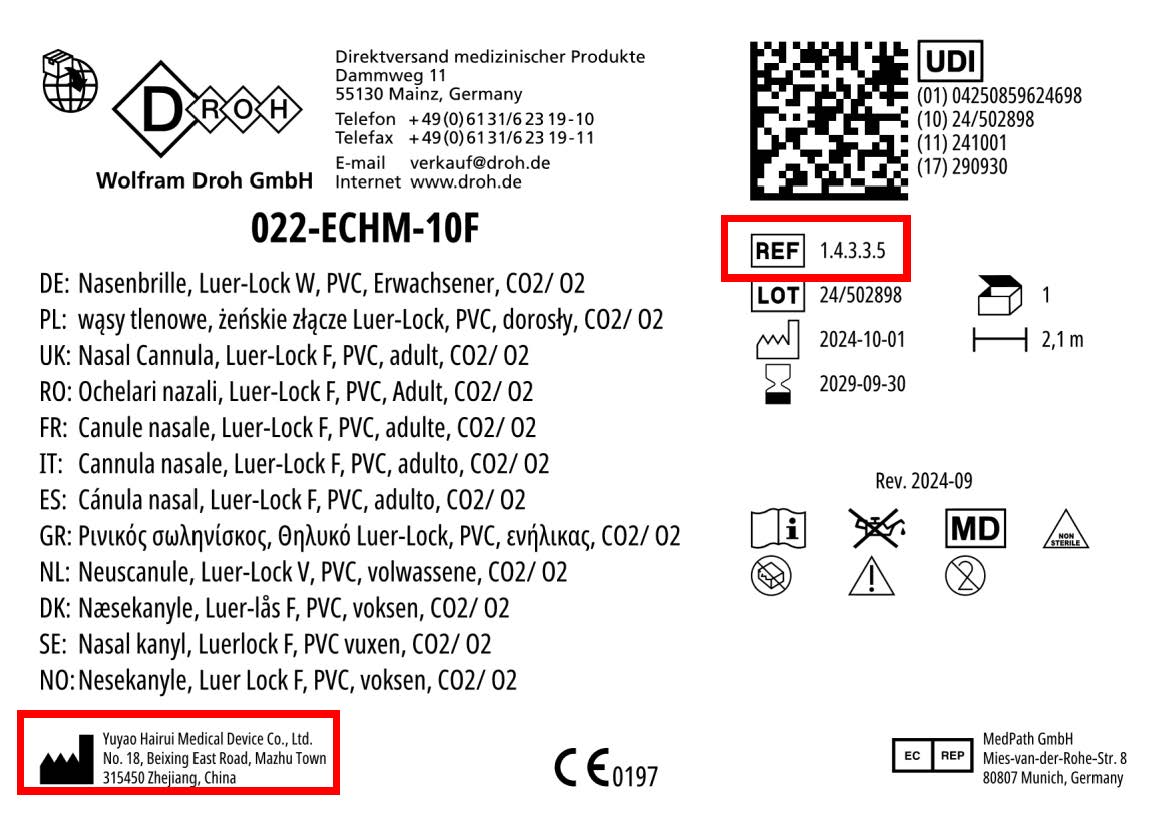
The Federal Office for Safety in Health Care (BASG) warns against counterfeit medical devices. The product "Nasal Cannula, Luer-Lock F, PVC, adult (REF 1.4.3.3.5)" has been counterfeited.
It cannot be ruled out that this product is also on the Austrian market. Counterfeit medical devices have no or an unpredictable effect and are to be classified as dangerous. They must not be used under any circumstances.
The product is a special tube system with a nasal cannula ("oxygen nasal cannula") to transport concentrated oxygen through the nose into the lungs.
The medical device manufacturer Yuyao Hairui Medical Device Co., Ltd. is mentioned on the packaging of the product. According to its own statements, it has never produced or marketed these products.
According to the manufacturer, the counterfeits can be recognised by the following feature:
On the product label, the manufacturer Yuyao Hairui Medical Device Co, Ltd, No.18, Beixing East Road, Mazhu Town 315450 Zhejiang, China is listed next to the manufacturer symbol for products with the reference REF 1.4.3.3.5 .
According to the manufacturer of the original product, the following risks exists:
Counterfeit products may be made of non-biocompatible plastics, which may cause skin irritation, allergic reactions or toxic exposure. There is no guarantee of proper sterilisation and therefore a risk of infection. Poor quality tubing can kink, tear or come loose, resulting in insufficient oxygen supply. An incorrect inner diameter or connector size can lead to insufficient oxygen supply, hypoxia or excessive pressure. The lack of appropriate testing can lead to unpredictable oxygen flow rates, posing a risk to neonates, elderly or critically ill patients.
Yuyao Hairui Medical Device Co, Ltd, is not the legitimate manufacturer of the counterfeit product and therefore not responsible for its production and composition.
The BASG asks you to report any suspicious activities in connection with the above-mentioned products by e-mail to the BASG(medizinprodukte@basg.gv.at) and the authorised representative of the manufacturer (MedPath GmbH, info@medpath.pro).
| Medical devices | Nasal Cannula, Luer-Lock F, PVC, adult REF 1.4.3.3.5 |
|---|